NanoFUSE™ – The Biologic Solution Trusted by the LESS Society
- sukanyarao
- Aug 7, 2025
- 3 min read
Updated: Aug 13, 2025
Written by Sukanya Chebrolu, MS and Chukwunonso C. Ilogu, MD, MS of the LESS™ Society.
In the evolving landscape of spine surgery, innovation is key to achieving better patient outcomes while minimizing risks. The LESS™ Society is at the forefront of this movement, advocating innovative technoloies that adhere to the Less Exposure Spine Surgery (LESS™) principles and following the REP philosophy: Restore motion, Early intervention and Preserve anatomy.
One groundbreaking osteobiologic technology leading this transformation is NanoFUSE™. It represents the future of spine treatment by optimizing bone fusion with minimal surgical impact.
What is NanoFUSE™? A Breakthrough in Biologic Spine Treatment
NanoFUSE™ is the only FDA-approved combination of synthetic 45S5 nano-bioactive glass and demineralized bone matrix (DBM). This unique formulation provides a powerful osteoconductive and osteoinductive environment, promoting natural bone regeneration (1). By leveraging advanced nanotechnology, NanoFUSE™ enhances spinal fusion while minimizing the need for invasive procedures, aligning perfectly with the LESS™ Society's mission (2).
Unlike traditional bone graft options that only work passively at the defect margins, NanoFUSE™ DBM is osteopromotive, meaning it actively stimulates new bone formation throughout the entire defect site. Clinical studies have shown a 290% increase in IGF-II growth factor expression, leading to greater osteoblast proliferation and bone regeneration. Compared to other commercially available DBM pastes, NanoFUSE™ DBM demonstrates superior performance with higher concentrations of osteoblasts, enhanced new bone formation, and increased presence of bone marrow.
For patients and surgeons looking for a bone graft solution that combines safety, effectiveness, and innovation, NanoFUSE™ DBM offers a proven, FDA-cleared option for achieving strong and reliable spinal fusion outcomes.
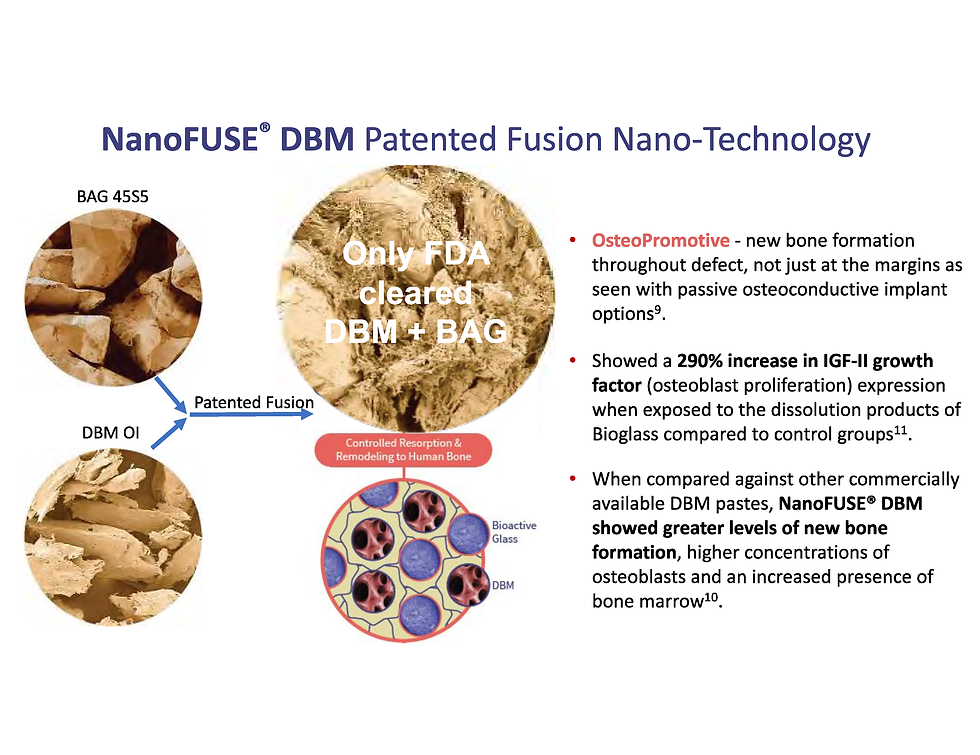
Image source: https://www.nanofusebiologics.com/science
How Does NanoFUSE™ Work?
NanoFUSE™ combines bioactive materials with the body’s natural healing mechanisms to stimulate bone growth. Acting as a nanotechnology-based bone graft substitute, it provides a scaffold for new bone formation while delivering essential growth factors to accelerate healing. This innovative approach improves surgical outcomes, reduces procedural invasiveness, and enhances recovery.
How NanoFUSE™ Supports LESS™ Spine Surgery
The LESS™ Society recommends the use of advanced biologics that enhance fusion success rates while minimizing surgical impact. NanoFUSE™ plays a pivotal role in this mission by:
Optimizing Bone Healing: Providing superior osteostimulation compared to traditional grafts.
Reducing OR Time: Its ready-to-use formulation streamlines surgical workflows, a key advantage in LESS procedures.
Enhancing Patient Outcomes: The combination of LESS™ surgery and NanoFUSE™ results in faster recovery and reduced post-operative complications (3).
Benefits of NanoFUSE™
For Surgeons:
Ease of Use: Seamlessly integrates into existing surgical workflows, reducing complexity.
Improved Outcomes: Advanced biologic properties support stronger, more reliable fusion results.
Reduced Complications: Promotes natural bone growth, minimizing the risk of graft rejection or failure.
For Patients:
Less Recovery Time: Less post-operative pain and a quicker return to normal activities.
Less Invasive: Supports LESS™ techniques, reducing tissue damage and scarring.
Long-Term Success: Ensures durable fusion results, reducing the need for follow-up surgeries.
For Healthcare Systems:
Cost-Effective: Reduces complications and hospital stays, lowering overall healthcare costs.
Enhanced Efficiency: Faster recovery times free up resources for other patients.
Frequently Asked Questions (FAQs)
1. What is NanoFUSE™?
NanoFUSETM is an advanced bone graft technology that combines demineralized bone matrix (DBM) with bioactive glass nanoparticles to enhance bone regeneration. It is used in spinal, orthopedic, and trauma surgeries to promote faster and more effective bone healing.
2. How does NanoFUSE™?
NanoFUSETM leverages the osteoinductive properties of DBM (which contains bone growth factors) and the osteostimulatory effects of bioactive glass, which releases ions that enhance cellular activity. This combination creates an optimal environment for new bone formation while maintaining structural integrity.
3. Is NanoFUSE™ FDA-approved?
Yes, NanoFUSETMT is FDA-approved for use in bone grafting procedures. It meets regulatory standards for safety and effectiveness in enhancing bone regeneration.
If you’re ready to take control of your spine health, visit NanoFUSE Biologics or reach out to the LESSTM Society for more information. Your journey to a pain-free future starts today.
References
Kirk JF, Ritter G, Waters C, Narisawa S, Millán JL, Talton JD. Osteoconductivity and osteoinductivity of NanoFUSE(®) DBM. Cell Tissue Bank 2013;14:33–44. https://doi.org/10.1007/s10561-012-9297-1.
Chin KR, Francis RR, Costigan WM, Spayde E, Ike C, Jeong Y, et al. Salvage of failed direct lateral sacroiliac joint fixation using a new percutaneous lateral-oblique transfixation technique with two variable-threaded screws: a multicenter case report of three cases. J Spine Surg 2023;9:348–56. https://doi.org/10.21037/jss-23-43.
Chin KR, Pencle FJ, Seale JA, Pandey DK. CT Scan and Clinical Outcomes of Novel Lateral-Oblique Percutaneous Sacroiliac Joint (SIJ) Fixation: Technique and Literature Review. Cureus 2021;13:e16408. https://doi.org/10.7759/cureus.16408.

Comments